Press Release
Warning on slimming product with banned drug ingredient
13 July 2012
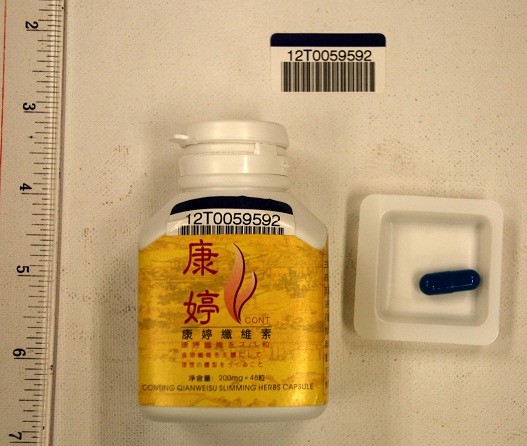
The Department of Health (DH) today (July 13) appealed to members of the public not to buy or consume a slimming product called "Conting Qianweisu Slimming Herbs Capsule", as it may contain banned drug ingredient that is dangerous to health. The appeal followed the DH's receipt of notification from the Hospital Authority (HA) about a 28-year-old lady who had a history of consuming a slimming product. The DH commenced investigation immediately.
A DH spokesman said, "The patient attended the Accident and Emergency Department of Caritas Medical Centre on July 8 because of chest pain and sweating. She described a history of consumption of the above slimming product. A drug-related adverse effect was suspected. She was discharged from hospital after treatment."
"The HA's laboratory test on the product sample showed the presence of the banned Western medicine sibutramine. Investigation showed that this product was purchased outside Hong Kong. DH's investigation continues."
"Sibutramine is a Part I poison and was once a Western medicine used as an appetite suppressant. Since November 2010, products containing sibutramine have been banned in Hong Kong because of increased cardiovascular risk," the spokesman explained.
People must stop using the product immediately and consult a doctor if they feel unwell or are in doubt after taking the product. The spokesman urged members of the public not to buy products of unknown or doubtful composition, or consume products from unknown sources.
"Weight control should be achieved through balanced diet and appropriate exercise. People should consult healthcare professionals before using any medication for weight control," the spokesman said.