Press Release
Warning on oral product containing undeclared Western drug ingredient
5 Apr 2012
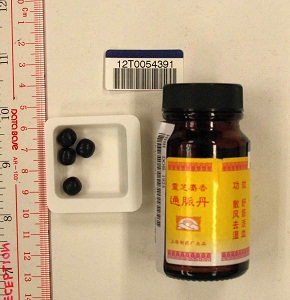
The Department of Health (DH) today (April 5) appealed to members of the public not to buy or consume an oral product called "Ling Zhi She Xiang Tong Mai Dan", as it may contain undeclared Western drug that is dangerous to health.
The appeal followed the DH's receipt of notification from the Hospital Authority (HA) about an 87-year-old male patient. The DH commenced investigation immediately.
"The patient was sent to the Accident and Emergency Department of Tseung Kwan O Hospital on March 5 because of gouty arthritis and decreased appetite. He was subsequently found to have features of Cushing's syndrome which can be caused by steroid overdose.A drug-related adverse effect was suspected. He is currently in stable condition.
"He described a history of consumption of an oral product called ‘Ling Zhi She Xiang Tong Mai Dan’ in the past one year. The HA's laboratory test on the product sample showed the presence of the undeclared Western medicine dexamethasone. Investigation showed that this product was purchased in Malaysia. DH's investigation continues," a DH spokesman said.
"Dexamethasone is a steroid. Taking dexamethasonefor a long time, especially when in substantial dosage, can cause side effects such as moon face, high blood pressure, high blood sugar, muscle atrophy, peptic ulcer and even osteoporosis," the spokesman explained.
"People who have taken the above product should consult healthcare professionals as soon as possible if they feel unwell or are in doubt. In addition, members of the public are urged not to buy or consume products of unknown composition or from doubtful sources. They should consult healthcare professionals whenever they feel unwell or are in doubt after having taken suspicious products," the spokesman warned.