Press Release
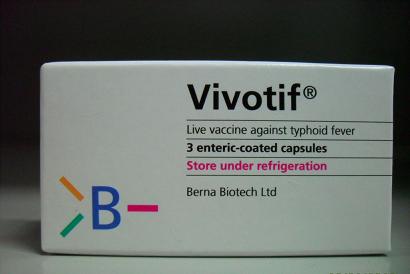
Recall of Vivotif typhoid fever vaccine
19 Jan 2011
The Department of Health (DH) has received notification from a licensed wholesaler, Amedis Company Limited, that it is conducting a voluntary recall of one lot of an oral typhoid fever vaccine, Vivotif Enteric Coated Cap (Registration Number HK-55557, Lot Number 3001862), as the Swiss manufacturer's stability monitoring showed a lower than expected potency value for the lot concerned.
The manufacturer confirmed that the problem is a quality issue and there is little risk to users as all lots on the market are still within the potency limit. The voluntary recall is just a precautionary measure.
Vivotif Enteric Coated Cap is an oral typhoid fever vaccine and a prescription drug. It can only be sold in dispensaries on doctor's prescriptions.
Investigation by DH revealed that 402 boxes of the affected product (Lot No.:3001862) had been sold to private hospitals, pharmacies and private medical practitioners.
The wholesaler has set up a hotline, 8100 8606, operating Monday to Friday between 9 am and 5 pm, to answer public enquiries.
DH is writing to healthcare professionals to alert them about the development.