Press Release
Slimming products found containing western drug ingredient
20 July 2007
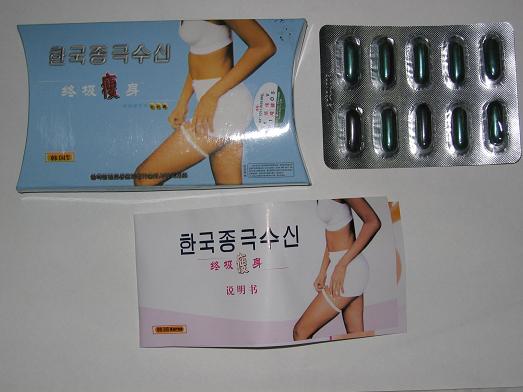
The Department of Health (DH) today (July 20) called on members of the public not to buy or use two brands of slimming products, one carried a Chinese name”終極瘦身” (named as J-minus終極瘦身on the Internet) and another called “Jelimel sliming capsules” (named as瘦身救兵on the Internet) because they were found to have contained sibutramine (西布曲明), a western drug ingredient that may cause side effect.
The appeal came after recent joint operations by DH and the Police which have resulted in the seizure of 79 boxes of終極瘦身at a premises in Tai Wai and 21 and 66 bottles of Jelimel sliming capsules from a shop in Mong Kok and a premises in Yuen Long respectively.
A man was arrested during the operations for終極瘦身and three women were arrested for Jelimel sliming capsules due to “Sale of Part I Poisons” and “Possession of Part I Poisons” under the Pharmacy and Poisons Ordinance. Investigation was still on-going.
A DH spokesman said a complaint against終極瘦身was received from a member of the public who suffered from symptoms of dry mouth, nausea and fast pulse rate after consuming the product purchased through an internet auction site. Her symptoms subsided after she discontinued with the capsules.
Another report was received from the Hospital Authority. Two women suffering from palpitation and tremor after taking Jelimel sliming capsules obtained from an internet auction site. The symptoms of the patients subsided after they stopped taking the capsules.
DH obtained samples of the two products for laboratory analysis and found that they both contained sibutramine, an appetite suppressant which can cause increased blood pressure and heart rate. Persons with heart diseases should not take it.
The spokesman said the slimming products concerned were not registered in Hong Kong and DH has no record of these products having been imported for sale. DH had asked the management of the concerned websites to remove the products from the auction lists.
The spokesman said under the Pharmacy and Poisons Ordinance, products containing sibutramine must be registered before sale and can only be sold on a doctor’s prescription and under supervision by a pharmacist.
People who have been using the products are advised to immediately stop taking them. They should seek medical attention if they feel unwell.
The spokesman said, “Members of the public are reminded that weight control should be achieved by observing good dietary practice and appropriate physical activities. They should consult their doctors before using any drug for weight control.”
Anyone who is in possession of the products should dispose of them or submit them to the DH’s Pharmaceutical Service at the third floor, Public Health Laboratory Centre, 382 Nam Cheong Street, Kowloon during office hours.