Press Release
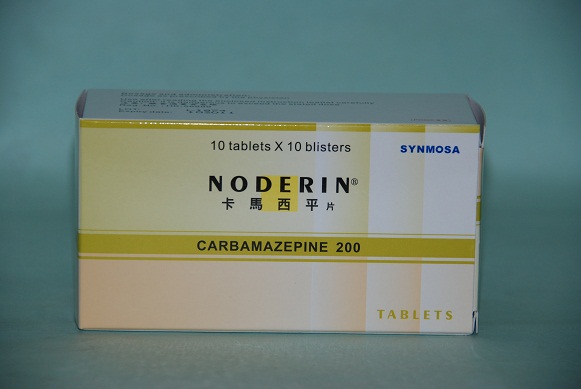
Total Recall of Noderin Tablet 200mg
3 May 2012
The Department of Health (DH) today (May 3) instructs a licensed drug wholesaler, Synmosa Biopharma (Hong Kong) Co. Ltd. ("Synmosa"), to conduct a total recall of Noderin Tablets 200mg ("Noderin") (registration no. HK-56889) from consumers due to quality defect.
DH was notified by Synmosa that the Taiwan manufacturer found one batch of the product failed the dissolution test. As the quality defect may affect the efficacy of the product, total recall from consumers is warranted.
Noderin contains carbamazepine and is indicated for epilepsy and trigeminal neuralgia. It can only be sold by prescription and under the supervision of pharmacists at registered pharmacies.
According to Synmosa, the product has been supplied to pharmacies and private doctors.
DH investigation continues.
So far, DH has not received any related adverse report.
Synmosa has set up a hotline 2708 9166 to answer related enquiries. DH will monitor the recall.
"Here, contravention of the Public Health and Municipal Services Ordinance (Cap 132), selling drug not of the nature, substance or quality demanded by the purchaser, might have occurred. The maximum penalty involved is $10,000 and 3 months' imprisonment," a DH spokesman remarks.
“Members of the public who have taken the product should not stop the medication but should seek advice from healthcare professionals ," the spokesman says.