Press Release
Recall of arsenic-tainted proprietary Chinese medicine
24 Jun 2011
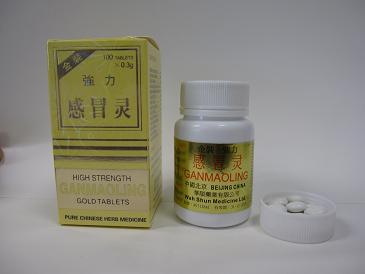
The Department of Health (DH) today (June 24) orders the wholesaler of a proprietary Chinese medicine, Wah Shun Medicine Limited, to recall from consumers a batch (batch no: 801128A1) of [W.S] Gan Mao Ling (registration no: HKP-00916) as it is found to contain excessive arsenic.
A spokesman from DH elaborates that the action is called for after the Government Laboratory detected about 1.7 times the permitted level of arsenic in a surveillance sample obtained from the market by DH pharmaceutical inspectors.
The drug is used to relieve symptoms of common cold and flu.
Initial investigations by DH reveals that the said batch of [W.S] Gan Mao Ling was manufactured in the Mainland and imported by Wah Shun in December 2009. It was then distributed to local retailers. No more batches have been imported since then.
The DH spokesman comments that arsenic is a heavy metal. While acute poisoning may cause severe vomiting, diarrhoea, confusion and coma, prolonged exposure can adversely affect the liver, kidney and heart. Young children are particularly vulnerable.
As investigation continues, the DH has instructed the company to recall the batch of product from consumers immediately.
The matter has also been referred to the Mainland's drug regulatory authority for their necessary follow-up.
Members of the public can call the wholesaler's hotline at 2571 0775 for related enquiries. DH will be monitoring the recall.
"Here, contravention of section 54 of the Public Health and Municipal Services Ordinance, Cap 132, Laws of Hong Kong, selling a drug intended for use by man but unfit for that purpose might have been occurred. The maximum penalty involved is $50,000 and six months imprisonment. On completion of the investigation, we will work with the Department of Justice for possible prosecution," the spokesman remarks.
The spokesman urges those members of the public who have purchased the product to stop using it immediately and surrender it to DH at 16/F, Two Landmark East, Kwun Tong, Kowloon.
"For those who have used the product and are feeling unwell, they should seek advice from their healthcare providers as soon as possible. So far, DH has not received any adverse report related to the product," the spokesman states.