Press Release
Woman arrested for allegedly selling slimming products with undeclared and banned drug ingredients
24 Jun 2011
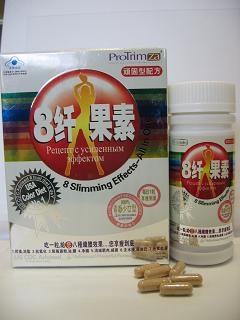
An 18-year-old woman was today (June 24) arrested in a joint operation by the Police and the Department of Health (DH) as part of their follow-up investigation into the sale of 2 slimming products, "8 Slimming Effects – All in One(青春少女型)" and "8 Slimming Effects – All in One(貴夫人型)", which were earlier found to contain undeclared and banned drug ingredients that may cause serious side effects.
In today's operation, the woman was caught for suspected illegal sale of the 2 products "8 Slimming Effects – All in One(青春少女型)" and "8 Slimming Effects – All in One(貴夫人型)", which are unregistered pharmaceutical products and contain Part I poison.
The investigation continues.
Earlier on, through the DH's surveillance programme, the affected products were discovered to be on sale on an Internet auction website and tested to contain undeclared and banned drug ingredients, sibutramine and its analogue, as well as phenolphthalein. Subsequently a press statement was issued asking members of the public not to consume the products.
A DH spokesman said the 2 products were not registered pharmaceutical products in Hong Kong.
Sibutramine is a Part I poison and was once a Western medicine used as an appetite suppressant. In November 2010, products containing sibutramine were banned because of increased cardiovascular risk. Phenolphthalein was once used for treating constipation but has been banned for its cancer-causing effect.
Sibutramine analogues, being chemically similar to sibutramine, are expected to have similar side effects as sibutramine.
The 2 products contain banned sibutramine or phenolphthalein and they are not allowed for sale in Hong Kong. Sale of an unregistered pharmaceutical product is an offence under the Pharmacy and Poisons Ordinance. The maximum penalty is a fine of $100,000 and two years' imprisonment.
The spokesman exhorted members of the public not to sell products of unknown or doubtful composition.
"People should stop using the products immediately. They should consult a doctor if they feel unwell after taking the product," the spokesman said.
They should submit them to the department's Pharmaceutical Service at 3/F, Public Health Laboratory Centre, 382 Nam Cheong Street, Kowloon, during office hours for their disposal.
The spokesman said, "weight control should be achieved through balanced diet and appropriate exercise. People should consult healthcare professionals before using any medication for weight control".