Press Release
DH raids retail shop for suspected illegal sale of unregistered pharmaceutical products
26 May 2015
The Department of Health (DH) today (May 26) raided a retail shop in Yuen Long for the suspected illegal sale and possession of unregistered pharmaceutical products labelled to contain Part I poison in a joint operation with the Police.
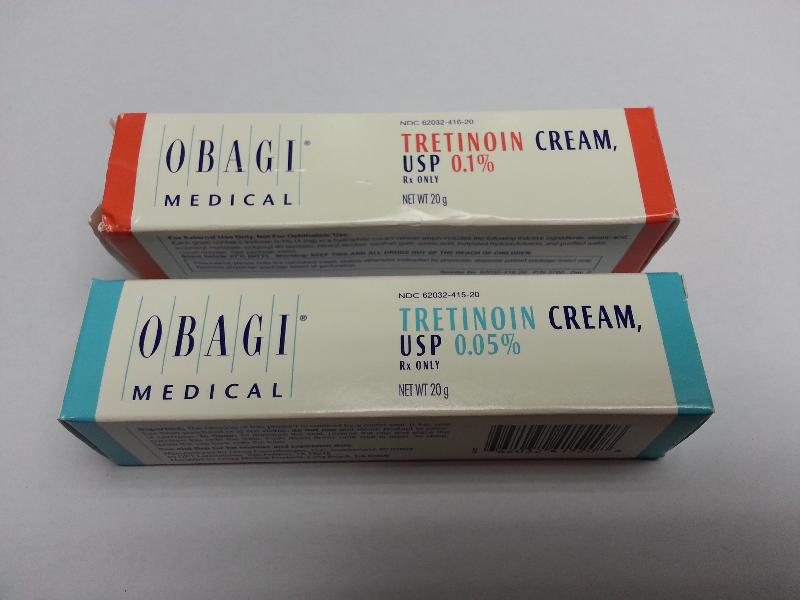
Acting upon a public complaint, it was found that the shop was offering for sale two cream products, namely Obagi Medical Tretinoin Cream, USP 0.1 per cent and USP 0.05 per cent, which were labelled to contain tretinoin, a Part I poison. Products containing tretinoin are prescription medicines which should only be used on the advice of a doctor or supplied at pharmacies under the supervision of a registered pharmacist upon a doctor's prescription.
A woman aged 20 was arrested by the Police for suspected illegal sale and possession of Part I poisons and unregistered pharmaceutical products in the operation.
The DH's investigation is ongoing.
Tretinoin is used topically for the treatment of acne. Its side-effects include erythema, dryness, peeling and photosensitivity. Sensitive individuals may experience oedema, blistering and crusting of the skin.
According to the Pharmacy and Poisons Ordinance (Cap 138), illegal sale and possession of Part I poisons and unregistered pharmaceutical products are criminal offences. The maximum penalty for each offence is a fine of $100,000 and two years' imprisonment.
A spokesman for the DH urged members of the public not to use controlled medicines on their own without advice from a doctor. They should not buy or use pharmaceutical products that are not registered. All registered pharmaceutical products carry a Hong Kong registration number on the label in the format of "HK-XXXXX". Safety, quality and efficacy of unregistered pharmaceutical products are not guaranteed.
People who have purchased the above products should stop using them and consult healthcare professionals if they are in doubt or feeling unwell after use. They can submit the products to the DH's Drug Office at Room 1856, Wu Chung House, 213 Queen's Road East, Wan Chai, Hong Kong, during office hours for disposal.