Press Release
Warning on slimming product with undeclared and banned drug ingredients
17 August 2012
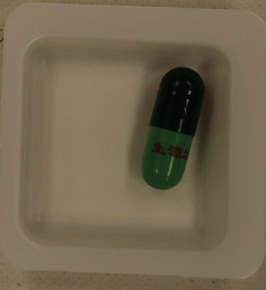
The Department of Health (DH) today (August 17) appealed to members of the public not to buy or consume a slimming product bearing the name "Sheng Yuan Fang" printed in Chinese on its green capsules, as it may contain undeclared and banned drug ingredients that are dangerous to health.
The appeal followed the DH's receipt of notification from the Hospital Authority (HA) about a 27-year-old lady who had a history of consumption of the above slimming product. The DH commenced investigation immediately.
"The patient was sent to the Accident and Emergency Department of Princess Margaret Hospital on August 8 because of loss of consciousness for a brief period. Metabolites of sibutramine and phenolphthalein were tentatively detected in her urine sample. Thus, a drug-related adverse effect was suspected. She was discharged from hospital on August 11 after assessment.
"The HA's laboratory test on the product sample showed the presence of an undeclared Western drug, sildenafil, and two banned Western drugs, sibutramine and phenolphthalein. Investigation so far revealed that the patient purchased the product from an internet site. The DH's investigation continues," a DH spokesman said.
In fact, DH previously identify similar products bearing the name of "Sheng Yuan Fang" adulterated with undeclared and banned western drug ingredients. Press releases were issued on 31 May 2010 and 24 February 2012 to alert the public not to consume the product.
"Sildenafil is usually used for treating male sexual dysfunction. The side effects of sildenafil include low blood pressure, headaches, vomiting, dizziness and transient vision disturbances. It may interact with some drugs (such as nitroglycerin for treatment of angina) and cause decrease in blood pressure to dangerous level. Improper use of sildenafil may pose serious health risks, especially for patients with heart problems.
"Sibutramine is a Part I poison and was once a Western drug used as an appetite suppressant. Since November 2010, products containing sibutramine have been banned because of increased cardiovascular risk. Phenolphthalein is another banned drug. It was once used for treating constipation but has been banned for its possible cancer-causing effect," the spokesman explained.
"Members of the public are urged not to buy or consume this product and must consult healthcare professionals as soon as possible if they feel unwell or are in doubt after taking the product. They should not buy or consume products of unknown composition or from doubtful sources," the spokesman said.
"Weight control should be achieved through balanced diet and appropriate exercise. People should consult health-care professionals before using any medication for weight control," the spokesman said.
"People who have the product should submit it to the department's Drug Office at 3/F., Public Health Laboratory Centre, 382 Nam Cheong Street, Kowloon, during office hours for disposal," the spokesman said.