Press Release
Total recall of unregistered Vimpat Tablets 50mg and 100mg
14 August 2012
The Department of Health (DH) today (August 14) instructed Orient Europharma Company Limited (Orient Europharma), a licensed wholesaler, to conduct a total recall of Vimpat Tablets 50mg and 100mg from the market as Orient Europharma had imported and distributed the unregistered version of the products containing soya lecithin coating instead of the registered version.
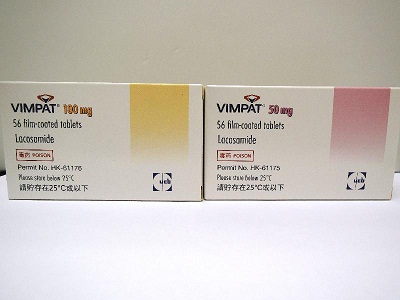
In Hong Kong, only Vimpat Tablets 50mg (registration number: HK-61175) and 100mg (registration number: HK-61176) without soya lecithin coating have been registered .
A DH spokesman said that UCB Pharma (Hong Kong) Limited, product certificate holder of Vimpat Tablets 50mg and 100mg, informed the DH today that their authorized importer, Orient Europharma, had imported and distributed the unregistered version of the products containing soya lecithin coating to the Hong Kong market.
According to the DH investigation so far, Orient Europharma imported 506 boxes of Vimpat Tablets 50mg and 497 boxes of Vimpat Tablets 100mg to Hong Kong in July this year. 11 boxes of Vimpat Tablets 50mg and 9 boxes of Vimpat Tablets 100mg were sold to local pharmacies and private doctors.
The DH's investigation is continuing.
Vimpat Tablets are manufactured in Germany by Schwarz Pharma Produktions GmbH and contain lacosamide for the treatment of epilepsy. They can only be sold on prescription and under the supervision of pharmacists at registered pharmacies.
"Here, contravention of the Pharmacy and Poisons Ordinance is suspected. The sale and possession of unregistered pharmaceutical products is an offence under the Pharmacy and Poisons Ordinance. The maximum penalty is a fine of $100,000 and two years' imprisonment," the spokesman explained.
Orient Europharma has set up a hotline (2578 7080) for the recall. The DH will monitor the recall.
"Members of the public who have the products in hand should not stop medication but should seek advice from healthcare professionals", the DH spokesman said.