Press Release
Warning on oral product containing banned Western drug ingredient
1 Jun 2012
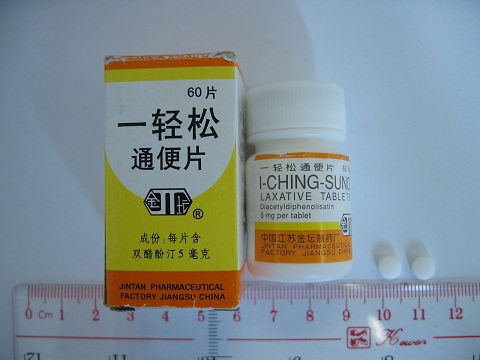
The Department of Health (DH) today (June 1) appeals to members of the public not to buy or consume an oral product called "Jin Tan 1-Ching-Sung Laxative Tablets", as it may contain banned Western drug that is dangerous to health.
The appeal follows the DH's receipt of information from the Hospital Authority (HA) about a 71-year-old female patient using a product containing banned Western drug. The DH commences investigation immediately.
"The patient sought treatment from the Accident and Emergency Department of Pamela Youde Nethersole Eastern Hospital on May 12 because of epigastric discomfort, right upper quadrant abdominal pain and passing tea-coloured urine. She was found to have deranged liver function. Incidentally she gave a history of use of the above product for two weeks in April this year. She is now in stable condition.
" The HA's laboratory test on the product sample showed the presence of the banned Western drug diacetyldiphenolisatin (also known as oxyphenisatin). Investigation showed that this product was not purchased locally. DH's investigation continues," a DH spokesman says.
"Diacetyldiphenolisatin was banned for its hepatotoxicity in Hong Kong in 1997. It was used previously for treating constipation." the spokesman explains.
The spokesman urges members of the public not to buy products from doubtful sources. People should consult healthcare professionals if they feel unwell or are in doubt after taking the product.
"They should submit it to the DH's Drug Office at 3/F, Public Health Laboratory Centre, 382 Nam Cheong Street, Kowloon, during office hours as soon as possible because it is an unregistered pharmaceutical product containing a banned drug ingredient, " the spokesman continues.
"Patients with constipation and any chronic disease ought to consult healthcare professionals for appropriate advice and management. They are strongly urged to refrain from self-medication or using over-the-counter products without professional supervision," the spokesman concludes.