Press Release
Press Release
Recall of two unregistered pharmaceutical products
1 Feb 2012
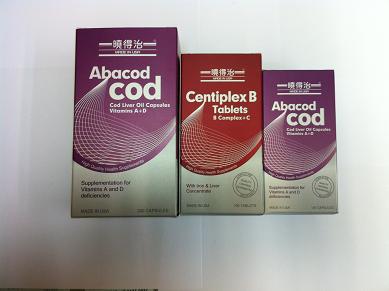
The Department of Health (DH) today (February 1) instructs Allways (Germany) Medicine Ltd. (Allways)to recall from the consumers two unregistered pharmaceutical products namely Centiplex-B Tab (HK-58444) and Abacod Cod Liver Oil Cap (HK-59342, 100’s and 300’s) as the products bear unapproved labels and render them unregistered.
A DH spokesman reveals that the matter came to light upon the department’s investigation into a public enquiry about the products.
Investigation reveals that Allways is the product registration certificate holder of the two products. However, the two unregistered products were sold to local pharmacies by W&S International Medicine Ltd (W&S) which is an unlicensed pharmaceutical product trader.
According to information obtained so far, Allways had not authorized any person to import and sell Centiplex B-Tab and Abacod Cod Liver Oil Cap 300’s. Allways imported unlabeled cod liver oil cap 100’s and transferred them to W&S.
DH is investigating into the sources of the unregistered products.
The unregistered products were packed with unapproved labels by W&S. As the safety and quality of the products could not be ascertained, recall from consumer is initiated as precautionary measures.
Centiplex-B Tab and Abacod Cod Liver Oil Cap are both vitamin supplement products.
So far, DH has not received any adverse event report in connection with the products.
“Here, contravention of the Pharmacy and Poison Ordinance and Import and Export Ordinance are suspected. The sale of unregistered pharmaceutical products is an offence under the Pharmacy and Poisons Ordinance. The maximum penalty is a fine of $100,000 and two years imprisonment. Import of pharmaceutical products without license is an offence under the Import and Export Ordinance. The maximum penalty is a fine of $500,000 and two years imprisonment," the spokesman explains.
Allways has set up a hotline 2412 5375 for public enquires. DH will monitor the recall.
"Healthcare professionals and retailers must stop supplying the products. Customers should also stop consuming the said products immediately. For those who have taken the affected products and are either in doubt or feeling unwell, they should consult their healthcare providers," the spokesman urges.