Press Release
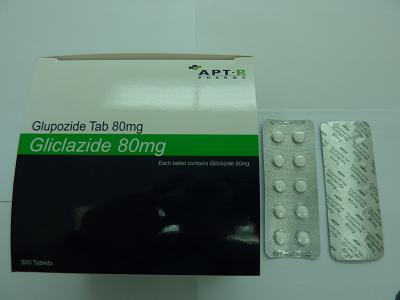
Recall of Glupozide 80mg tablet
28 Nov 2011
The Department of Health (DH) today (November 28) instructs a licensed drug manufacturer, APT Pharma Limited (APT), to recall from shelf a batch (Lot H00406) of Glupozide 80mg tablet (HK-48195) in view of a quality defect identified by DH through follow-up on a public complaint.
“As DH investigates into a user's complaint, we confirmed the finding of a suspected plastic fragment embedded in one of the above tablets,” a DH spokesman reveals.
“So far, this is an isolated detection, with no indication that other batches are affected or any report of related adverse incident," the spokesman comments. But investigation is still ongoing.
According to details provided by APT, the tablets were usually manufactured by APT Pharma (China) Co. Ltd in Guangdong and then exported to Hong Kong for packaging by APT.
"To be precise, the affected batch was manufactured in Guangdong in June this year. After packaging here, a total of 2 255 boxes of 500 tablets were supplied to various HA hospitals," the spokesman discloses.
Glupozide 80mg tablet, containing gliclazide, is used for the control of blood glucose in diabetic patients. It requires a doctor’s prescription and can only be sold in a pharmacy under the supervision of a pharmacist.
DH has already alerted the Hospital Authority and the Guangdong drug regulatory authority for their necessary follow-up.
APT has also set up a hotline 2341 7878 for public enquiries.
“Here, contravention of the Public Health and Municipal Services Ordinance (Cap 132), selling drug not of the nature, substance or quality demanded by the purchaser, might have occurred. The maximum penalty involved is HK$10,000 and three months’ imprisonment. DH will seek advice from the Department of Justice on completion of the investigation,” the spokesman states.
“Meanwhile, members of the public who are taking the above medicine should check the tablet, even splitting it for examination if necessary, before use. However, balancing the risks versus benefits, they must not stop using the drug abruptly, but instead ought to seek healthcare professionals' advice if in doubt or feeling unwell,” the spokesman urges.