Press Release
Voluntary Recall of suspected non-sterile 0.3% Potassium Chloride and 0.9% Sodium Chloride IV Infusion
03 Aug 2011
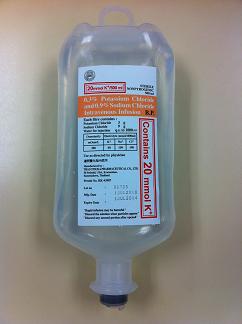
The Department of Health (DH) today (August 3) endorses a licensed drug wholesaler, Luen Cheong Hong Ltd (LCH)'s voluntary recall of a batch of pharmaceutical product called "0.3% Potassium Chloride and 0.9% Sodium Chloride IV Infusion" (Registration number: HK-43987) (Batch: OG705) after the user, the Hospital Authority (HA), detected failure in the preliminary sterility test performed as part of a routine in-house quality assurance programme. Neither HA nor DH has received related adverse event report.
"0.3% Potassium Chloride and 0.9% Sodium Chloride IV Infusion is used for replenishment of electrolytes in patients," a DH spokesman states.
"According to LCH's sales record, the affected batch OG705 was imported from Thailand into Hong Kong last year. Apart from a lot of 400 units which was re-exported to Macao, the batch was otherwise supplied solely to HA hospitals," the spokesman continues.
"The implication of a failed sterility test is that the infusion might have microbial contamination. Thus, it is prudent that the wholesaler recalls from end-users or else it would risk infecting patients if administered. In any case, DH would investigate into the matter, including conducting microbiological surveillance on samples of the infusion," the spokesman explains.
"It is also helpful that LCH has suspended trading of the infusion in order to facilitate our investigation," the spokesman remarks.
Luen Cheong Hong has set up a hotline at 2575 4486 to answer related enquiries.
The spokesman urges all healthcare providers who have the above quoted stock in hand to stop supplying the product any further and get in touch with the wholesaler as soon as possible. They should also report suspected adverse incidents to DH.
"In connection, members of the public should also consult their healthcare providers when in doubt and in particular if they feel unwell after having used the product," the spokesman advises.