Press Release
Voluntary recall of anti-acne cream
28 Jan 2011
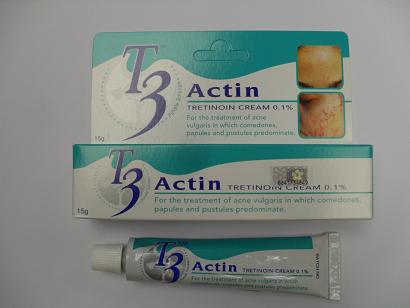
The Department of Health (DH) today (January 28) endorsed the voluntary action of a licensed drug wholesaler, Hoepharma (HK) Ltd, of recalling all batches of its T3 Actin Cream 0.1% (Registration No.: HK-57658) as Singapore has found that the content of its active ingredient Tretinoin is less than stated.
DH also noted the matter from its pharmacovigilance programme. Singapore's drug regulatory authority, Health Sciences Authority (HSA) detected the above defect and has ordered recall of the product over there.
A spokesman for DH said that deviations from the registered amount of active ingredient(s) of a pharmaceutical product reflected quality deficiency which could also affect treatment effectiveness. While the Department has collected samples for analysis, Hoepharma voluntarily recalled all products from the local market in order to be prudent.
T3 Actin Cream 0.1%, manufactured in Malaysia, is a prescription drug used for acne treatment. It can only be sold in pharmacies under the supervision of pharmacists and on doctor's prescriptions. It is known that Hoepharma has also supplied the cream to private doctors.
The wholesaler has set up a hotline 27109619 to answer public enquiries between 9 am and 5 pm during weekdays from Monday, January 31 onward.
The spokesman urged that healthcare professionals and retailers must stop supplying the said product to their clients. People who have used the affected product are advised to consult healthcare providers if in doubt.
The DH will liaise with HSA for further details while continuing with the investigation and monitoring of the recall.