Press Release
Caution against product containing undeclared western medicine
9 Nov 2010
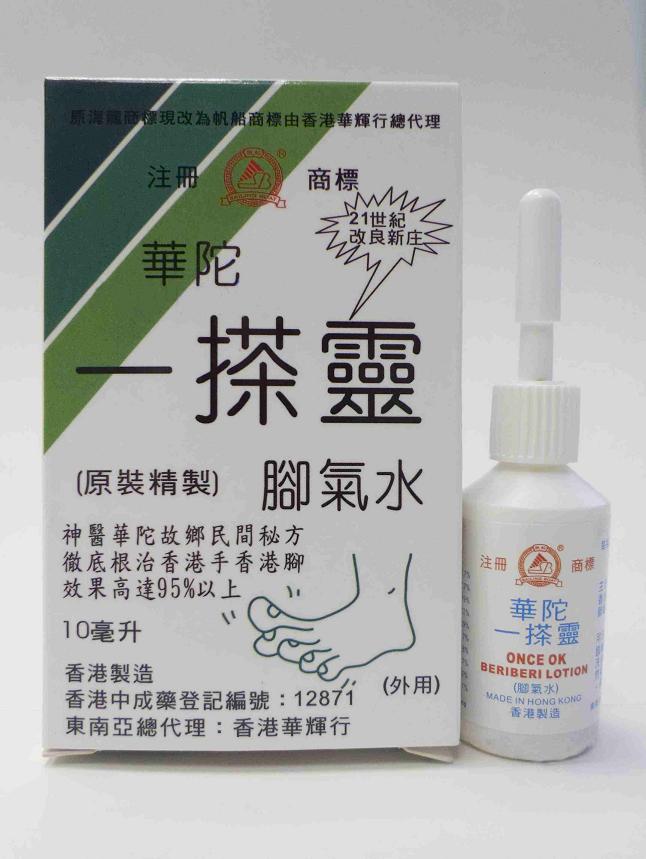
The Department of Health (DH) today (November 9) called on members of the public not to buy or use a topical product labelled as "Once OK Beriberi Lotion" as it was found to contain an undeclared western medicine, Benzoic acid, a drug used in treating fungal infections.
The appeal was made following DH's market surveillance under its established vigilance system, which aims to scan for possibly adulterated consumer products with health claims.
The product was manufactured in Hong Kong by licensed Chinese medicine manufacturer, Hong Kong Chung Shun Medicine Manufactory, and distributed by Wah Fai Hong, a licensed Chinese medicine wholesaler.
The wholesaler was instructed to immediately recall the product. It has set up a hotline (2415 2137) to answer public enquiries.
Investigation by DH continues.
Under the Pharmacy and Poisons Ordinance, products containing Benzoic acid for medicinal use must be registered before sale on the market.
Meanwhile, sale of unregistered pharmaceutical product is an offence liable to the maximum penalty of a $100,000 fine and two years' imprisonment.
Members of the public who have bought the product are advised not to use it. They are urged to seek advice from their healthcare professionals if feeling unwell after using the product.
They may destroy and dispose of the product or submit it to the department's Pharmaceutical Service at 3/F, Public Health Laboratory Centre, 382 Nam Cheong Street, Kowloon, during office hours.