Press Release
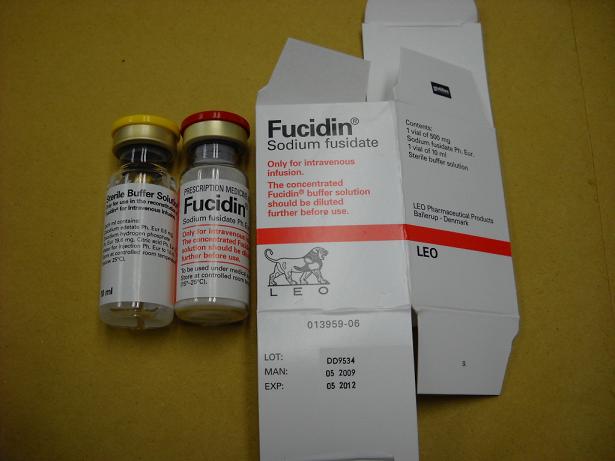
Voluntary recall of Fucidin for Intravenous Infusion due to quality defect
12 July 2010
The Department of Health (DH) was today (July 12) informed by licensed distributor DKSH Hong Kong Limited that manufacturer Leo Pharma is conducting a voluntary recall of all batches of Fucidin for Intravenous Infusion 500mg (Registration Number HK-37409) from the market in view of its quality defect.
The recall was conducted after the manufacturer received three complaints from the Mainland regarding the presence of glass fragments in some of the vials of the product. No report of patient injury has been received.
Fucidin for Intravenous Infusion 500mg, containing sodium fusidate, is an antibiotic which is available only on prescription and usually used in hospitals for various infectious diseases. The product was imported from Denmark.
According to Leo Pharma, the glass fragments identified in the product so far have a size too large to pass through most needles used for administration. The recall was initiated to eliminate any potential risks for patients.
Based on the distributor's information in hand, the affected product has been supplied to Hospital Authority hospitals and some private hospitals in Hong Kong. The relevant hospitals have been informed about the recall.
A DH spokesman said the department endorsed Leo Pharma's decision to recall the product to safeguard patient safety and will closely monitor the exercise and developments.
DKSH Hong Kong Limited has set up a hotline, 2895 9668, for public enquiries.
The spokesman urged healthcare professionals to stop administering the product to patients. "Patients should consult healthcare professionals if in doubt," he said.