Press Release
Recall of pharmaceutical products
14 Apr 2010
Subsequent to the incidents of recall of pharmaceutical products manufactured by the licensed drug manufacturer Neochem Pharmaceutical Laboratories Ltd in March 2010, the Department of Health's (DH) further investigations today (April 14) confirmed that two more products were found to contain lower than registered content of their active ingredients.
The manufacturer has initiated a recall of the affected batches of the two products.
Since the incident of recall in mid-March 2010, the manufacturer has suspended its production to facilitate the DH's targeted review on the quality issues of the manufacturer. The review has been completed.
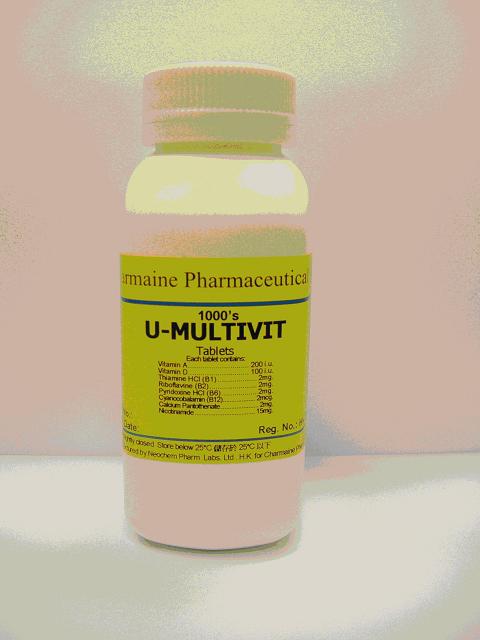
The products being recalled were U-Multivit Tab (Registration Number HK-28668, Batch Number 070756) and another batch of U-Caine Lozenges (Registration Number HK-27990, Batch Number 071382), both of which were found to contain lower than registered content of their ingredients. Another batch of U-Caine Lozenges (Batch Number 081228) was recalled at the end of March 2010.
According to the label of U-Caine Lozenges, each lozenge should contain 1mg of benzocaine. Analysis so far showed that the batch (Batch number 071382) of the concerned product only contained 0.36mg per lozenge.
The product was only sold in bulk pack. Based on information in hand, a total of 233 bottles (500 lozenges per bottle) of the affected batch had been supplied mainly to private doctors and some pharmacies.
As to the label of U-Multivit Tab, each tablet should contain Vitamin A at 200 International Units (I.U) and Vitamin D at 100 I.U. Tests made no detection of Vitamin A nor Vitamin D.
Based on information in hand, a total of 204 bottles (1,000 tablets per bottle) of the vitamins had been supplied to private doctors and some pharmacies.
U-Caine Lozenges are used for relief of sore throat and should be sold in pharmacies under the supervision of pharmacists. U-Multivit Tab is a Vitamin supplement.
A spokesman for DH said this was a quality issue but may affect the effectiveness of treatment.
The manufacturer has set up a hotline, 3427 3525, to answer clients' enquiries.
People who have sore throat not relieved or aggravated after taking U-Caine Lozenges are advised to consult healthcare professionals.
The spokesman said the DH would closely monitor the recall.