Press Release
Recall of pharmaceutical product with wrong label
19 Feb 2010
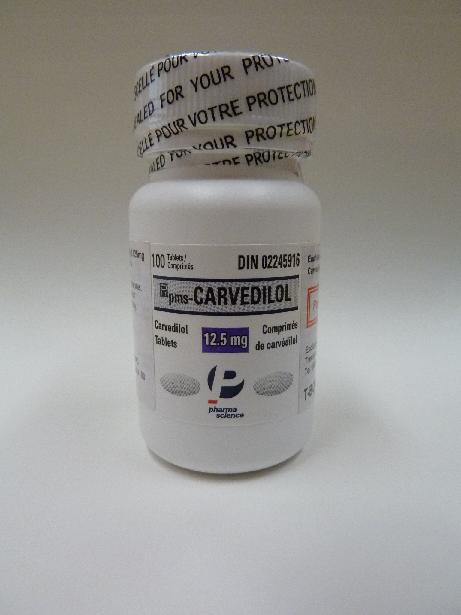
A spokesman for the Department of Health said today (February 19) that Trenton-Boma Ltd, a licensed wholesaler of pharmaceutical products, had initiated a recall of one batch of PMS-Carvedilol 12.5mg tablets (registration no. HK-52639).
The batch number of the product being recalled is 444937.
The product is manufactured in Canada. It is used for the treatment of high blood pressure and heart failure. It is a prescription-only medicine.
A total of 2218 bottles (100 tablets per bottle) of the batch had been supplied to the Hospital Authority, 71 bottles to some private doctors and pharmacies.
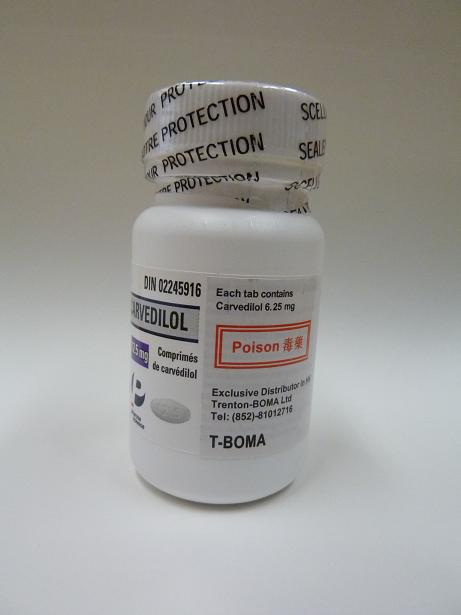
Based on information available so far, the recall is initiated because, on some of the bottles of the product, the additional label, which the wholesaler has added in order to comply with the local statutory labelling requirements, states incorrectly that each tablet contains 6.25mg carvedilol and shows an incorrect registration number.
The wholesaler has initiated a recall exercise at retail level. It has also set up a hotline (8101 2716) to answer clients’ enquiries.
Patients using this product should immediately consult their doctors if in doubt.
The DH will closely monitor the recall exercise. Investigations are in progress.