Press Release
Recall of gels for mouth ulcer
19 May 2009
A spokesman for the Department of Health today (May 19) said that Prowin Laboratories Ltd had initiated a voluntary recall of two batches of gels for mouth ulcer.
The spokesman said the recall was made after Prowin Laboratories Ltd reported to DH today that two products, Jalogel-1 Gel (batch number LX0699) and Jelaplexin Gel (batch number JW1969), had failed the limit level of bacteria and moulds in a recent review by the company. However, no pathogen was found.
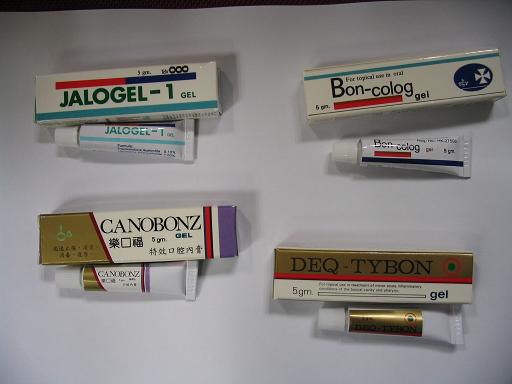
The two batches of the two products were packed under 13 different products (see annex ), with seven in batch number LX0699 and six in batch number JW1969.
Jalogel-1 Gel contains antibiotics and steroids and should be prescribed by doctor. Jelaplexin Gel contains mainly salicylate. Both were applied topically for pain- relief and as anti-inflammatory for mouth ulcers.
The company had initiated a recall from the market of 13 products of the two batches, which were contract manufactured by Marching Pharmaceutical Ltd.
DH immediately conducted investigation and found that there were a total of 9,884 tubes of gels manufactured in batch LX0699 and 9,910 tubes in batch JW1969. All of them were sold to medicine retailers in Hong Kong and for export to Macau.
DH has informed doctors and pharmacists about the recall.
Doctors, pharmacists and retailers are advised to stop administering and supplying them to their clients.
Members of the public are advised to stop using the products and seek medical consultation if they feel unwell.
Prowin Laboratories Ltd has set up a hotline (2425 5199) for enquiries.
DH's investigation is on-going.